Facts about atoms for kids
This article will tell you how to explain the basics of chemistry to a kid without scrambling brains.
What is an atom?
Every matter consists of atoms: the tiny particle of dust and entire planets. They are called principal construction materials for all living things.
The main property of atoms is indivisibility. Imagine an apple. We can easily cut it on two halves, then on quarters. But what if we continue to cut it on the smallest parts until you can not split it any further? So, a particle that we cannot divide further is suggested to be indivisible. And this is an atom.
How small is an atom?
They are so tiny that even a baby child consists of trillions of atoms! There are various kinds of atoms, each of them has its characteristics — size, mass, and name. Scientists measure the size of atoms in nanometers. It varies from 0,1 to 0,5 nanometers in width. The only way to see atoms is experimental. Scientists use special tools and lead experiments to watch how atoms work.
Just imagine: if we were the size of an atom, we would be smaller than a human hair! An ant would probably be seen as a dinosaur for us! Instead of traveling to different countries and even planets, traveling just a few centimeters would be a huge achievement!
Atoms and molecules
The group of atoms is called molecules. These building blocks work together and create multiple constructions. Depending on combinations of atom’s kinds, they create various substances — liquid, gasses, and solids. Look at the example below:
Two hydrogen atoms + one oxygen atom = molecule of water.
There is a shining example to display atoms and molecules to kids through sentences and letters that they consist of. The sentence “We are young chemists” looks like a molecule, as it is a group of words. But this sentence consists of letters — the smallest parts of it. The smallest and indivisible like atoms!
Сhemical elements
Elements are chemicals that contain an atom of one kind. The total of them is 118, but only 94 occur naturally on Earth. Chemical elements are of different origin:
- Some exist in pure form (gold, silver). They are called natural;
- Others are separated from other substances (for example, iron);
- 24 elements are synthetic. Researchers make them in a laboratory.
Tip to note: periodic table displays all known elements and arranges them on several characteristics: atomic number, electron configuration, and chemical properties.
Structure of an atom
Like a person has a skeleton, muscles, and skin, an atom has its construction. If you look at the illustrations of its structure, you will see:
- center or nucleus;
- so-called cloud that surrounds the center.
What do nucleus and cloud consist of?
Nucleus contains smallest particles — protons and neutrons;
Electrons are particles outside the center. They are orbiting the nucleus. Electrons make a so-called cloud around it.
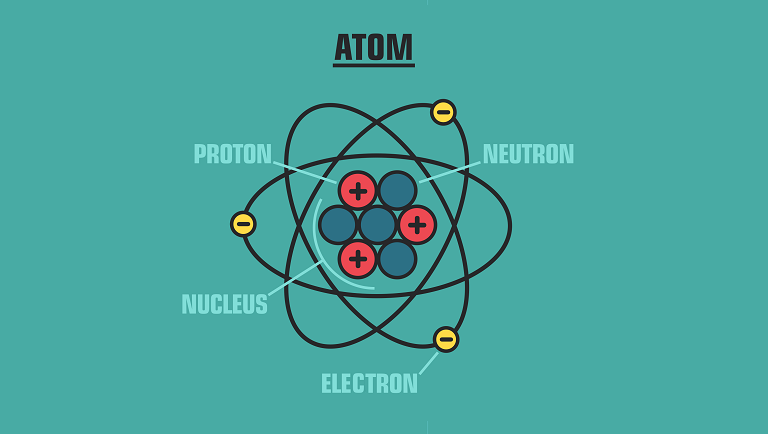
Takeaway: Atom’s body consists of three small elements — protons, neutrons, electrons. The nucleus contains protons and neutrons. This nucleus is surrounded by a cloud where electrons live.
Subatomic particles
Let’s define particles that atom contains:
- Proton is in the center of an atom that has a positive charge and mass.
- Neutrons are in the center too. Neutrons have no charge.
- Electrons are the smallest particles. They have a negative charge.
Tip to note: Positively charged protons attract negatively charged electrons due to electromagnetic force. Electrons orbit the nucleus but cannot breakthrough.
Fact: electrons spin around the atom incredibly fast. Scientists can only predict their place but can never be sure of it.
Even smaller: quarks and neutrinos
In the 20th century, scientists went deeper into intangible atoms. Their discoveries have widened knowledge about particles.
The researchers used particle accelerators. During their experiments, electrons fired at the protons. As a result, they bounced off tiny particles inside protons. The discovery of new particles has taken four years of experiments.
Their experiments discovered that scientists have an opportunity to split protons and neutrons. They found out that protons and neutrons consist of particles known as quarks.
Tip to note: quarks are named fundamental particles because they have no substructure and are indivisible.
Takeaway: quarks are tiny particles that make protons and neutrons. Quark is so small that it is impossible to detect. For many years nobody can figure out that they can exist, but in 1964 intangible particles were finally detected thanks to the particle accelerator.
Neutrinos are often called natural ghosts or even vampires of physics. It is a challenge to find them. In the 20th century, chemists had finally found them due to extraordinary experiments.
They are elementary particles with the smallest mass. Some facts about neutrinos:
- These particles have not electric charge. The word Neutrinos means Small Neutral Particles;
- Neutrinos interacts through gravitation and weak force;
- As they have no interaction through electromagnetic forces, it is impossible to see them;
- They move at the speed of light;
- They penetrate through solids.
Fact: every square centimeter of your skin gets hit with 65 billion neutrinos per second.
Mass number and atomic weight
There are several principal characteristics of chemical elements:
The atomic number is the total of protons that live in the center of an atom. It is principal property. It shows how many protons the atom has in its center. This characteristic defines the kind of an atom.
In the periodic table, this number appears above the element symbol. Look at Hydrogen as an example. It comes first in the periodic table as it has one proton.
Scientists can measure atoms in grams, but it is much easier to use an unconditional measure, as atoms are so tiny. So, there is an atomic mass. It defines how much one element is heavier or lighter than another one.
The number below the element’s symbol points at atomic mass. In theory, mass is the total of protons, neutrons, and electrons. But in practice, electrons are so small that it is hard to define their weight. Therefore, an atomic mass is considered to be the sum of protons and neutrons only.
The mass number is the number of protons and neutrons divided at a certain number that chemists agreed. Mass numbers are always whole numbers.
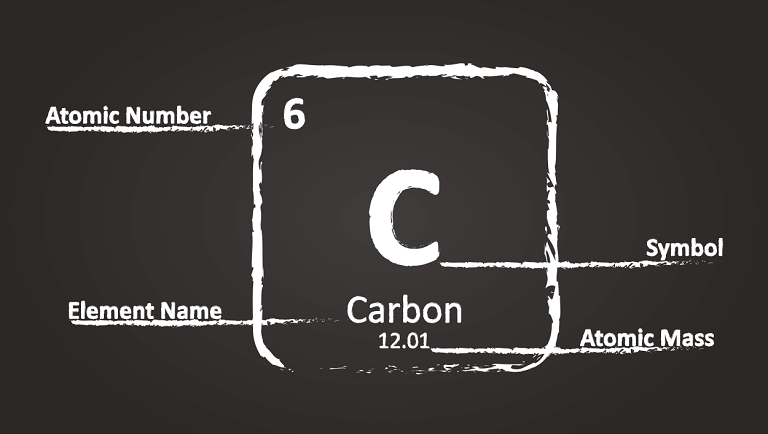
Let’s look at the carbon to watch its properties:
- Atomic number = 6. That means carbon has six protons.
- Mass number = 12. This figure shows that element carbon contains six protons and six neutrons.
- Atomic mass = 12.01
What are isotopes?
As we have mentioned above, the number of protons determines the chemical element. As protons have a positive charge, electrons have a negative one. The amount of them is always equal.
The number of protons identifies the number of electrons. But most elements in nature consist of atoms with different numbers of neutrons. They are called isotopes.
As you remember, the neutron is a particle without an electric charge. For this reason, this subatomic particle never changes elements. However, changing the number of neutrons affects their mass.
- Carbon-12 is an element with six protons and neutrons per atom.
- Carbon-14 has six protons and eight neutrons.
Tip to note: The carbon will never change the number of its protons. If not, carbon would not be carbon!
Ions, cations, anions
In ordinary conditions, each atom contains an equal sum of protons and electrons. They have positive and negative charges balanced thanks to an identical total of protons and electrons. This balance makes an ordinary atom neutral.
However, some atoms can lose electrons or gain additional ones due to their interaction in chemical reactions or collisions with different particles. As a result, the atom transfers into a new particle with a positive or negative charge.
Takeaway: Ions are particles that have a positive or negative charge.
When an atom gets rid of one or more electrons, the amount of protons becomes dominant, and it shifts to the positive charge. It is a cation.
When an atom gains one or more electrons, the total of electrons becomes dominant. Therefore, an ion becomes negatively charged. This type of ion is called an anion.
Radioactive decay
Most elements are always stable. That means that atoms taking part in various chemical reactions will never change themselves, but not always. Some atoms have too big nuclei — too many protons and neutrons. This nucleus is called unstable. An unstable nucleus always tries to get rid of excess particles through radiation. Unstable atoms are suggested to be radioactive until they dispose of excess mass.
Tip to note: all chemical elements above atomic number 82 are radioactive. Bananas and rocks are things with very little radiation. Therefore, they are not harmful. For example, sunburn can be dangerous due to sun radiation.
Radioactive decay is a name of a phenomenon of changing atoms with unstable nuclei. There are three types of radiation decay.
Alpha decay
It is a phenomenon when an atom gets rid of two protons and two neutrons.
For example: when beryllium with atomic number 4 undergoes radiation decay, it will become helium with atomic number 2.
Betta decay
When an atom undergoes betta decay, its protons turn into neutrons, or its neutrons turn into protons. As a result, it becomes an element with one atomic number lower or higher.
Gamma decay
In that case, an atom doesn’t change atomic number or mass. It shoots out gamma waves.
Who discovered atomes
The first time scientists thought of this mysterious particle in the Ancient period. Greek philosophers called this strange thing Atomos, which means “indivisible”. Thus, ancient Greeks first pointed at the significant property of the atom. But it has taken more than 1800 years to understand how these basic particles work.
Summary
- Atom is an extremely tiny particle;
- It consists of three particles in the atom’s structure;
- Protons are particles with a positive electrical charge;
- Neutrons don’t have any electrical charge;
- Electrons are negatively charged particles that orbiting the nucleus;
- Isotopes are elements that contain atoms with different number of neutrons;
- Quarks are fundamental particles that cannot be split. Protons and neutrons contain quarks;
- Ions are particles with an unequal number of protons and electrons;
- Chemical elements above atomic number 82 are all radioactive.
You may also be interested:
States of matter: solids, liquids and gases — simple explanation for kids

new engaging articles