Periodic table of chemical elements for kids
What is the Periodic table of chemical elements?
The Periodic table of chemical elements is a structured table with a sequence of chemical elements arranged in strict order with the periodic law of chemistry.
This is a clear example of the periodic law, which was formulated by Dmitry Ivanovich Mendeleev in 1869. Nowadays, the formulation of this law is as follows: the elements of the table have changes in their properties in accordance with certain periods.
In order to realize the structure of the periodic table of chemical elements, you must first understand what a chemical element is.
What is a chemical element?
A chemical element is a specific type of atom with an individual set of protons and neutrons in the nucleus and electrons that revolve around the nucleus. Atoms of different chemical elements differ in mass, size, structure, and properties. Each chemical element has a name and is indicated by a symbol or chemical sign. The symbol for a chemical element consists of one or two letters. As a rule, the first letters of its Latin name are used. For example, Nitrogen is indicated as “N”, Aluminum as “Al”, Helium as “He”, Iodine as “I”.
To date, 118 chemical elements are known. 94 of them are found in nature (some only in trace amounts), and the others 24 are artificially synthesized.
Atoms of various elements interact to form compounds in different combinations and form a huge amount of natural and synthetic substances.
Why do we call the table “periodic”?
But let’s get back to the periodic table of chemical elements. Why is it called “periodic”? The answer to this question is straightforward.
If you look closely at the structure of the table, you will notice a periodic change in the properties of the elements with increasing nuclear charge. And the nuclear charge, in turn, is the number of a chemical element in the table. It is always positive.
For example, hydrogen has a nucleus charge of +1, calcium will have +20, and so on. Also, the elements in the table are ordered according to the electronic configuration of the atoms and the repeated chemical properties. In the table, periods are rows, and groups are columns.
Who discovered the periodic table of chemical elements?
By the middle of the 19th century, scientists had already discovered 63 elements from the modern periodic table. At that time, all chemists of the world tried to structure all known elements into a single concept. They attempted to create a sequence of elements in increasing order of atomic mass and then separate them into groups due to similar chemical properties.
But the first scientist who was able to combine and organize all the knowledge accumulated by humanity about chemical elements into a structured table was the Russian scientist Dmitry Ivanovich Mendelev. Mendeleev published his first sketch of the periodic table in 1869 in the article “Correlation of properties with the atomic weight of elements” (in the journal of the Russian Chemical Society). Even earlier (February 1869), the scientific notice of the discovery was sent to the world’s leading chemists.
According to Mendeleev, there should be elements with the same valence in one column. So he decided to leave empty cells in his table while carefully studying the dynamics of the increase in atomic weights. Then he correlated this with the valencies in typical compounds and the chemical properties of the elements.
At first, Mendeleev’s colleagues reacted with suspicion to his theory of missing elements. Still, within 15 years, new elements such as gallium, scandium, and germanium were discovered. Their properties exactly matched the characteristics described by Mendeleev. After that, skeptics had no doubts about the significance of the Periodic table.
How to read the Periodic table?
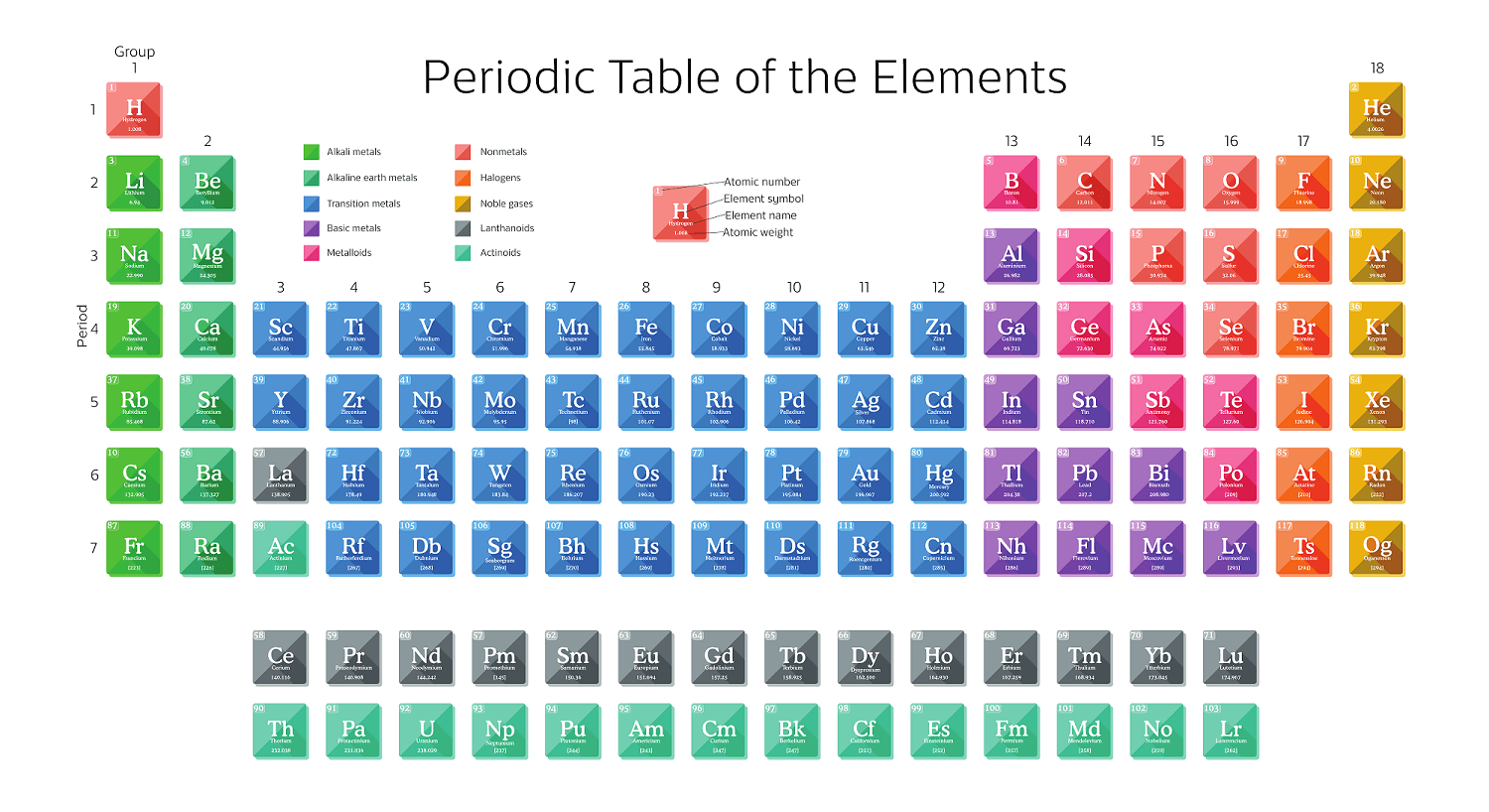
Anyone who wants to understand chemistry should know what information is contained in Mendeleev’s periodic table, what stands for each number and symbol in each cell.
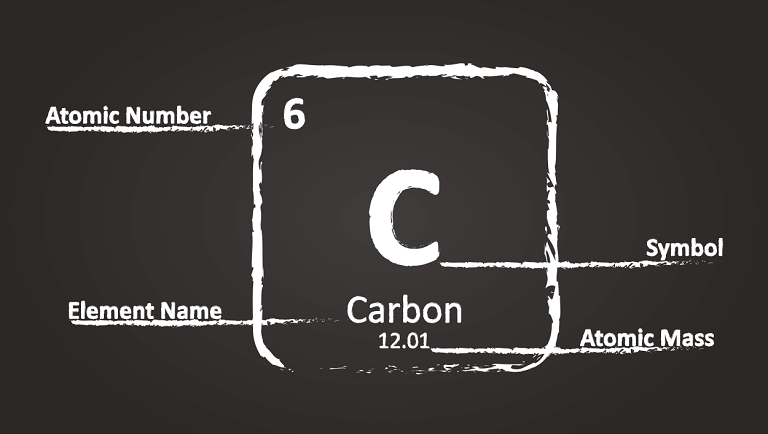
Let’s start with the ordinal number of a cell. It is located in the upper right corner of this cell. This number tells us two things. First of all, it coincides with the number of protons in the nucleus of a given element. That is, there will be seven protons in the nucleus of the atom of the seventh element.
If there are ninety-two protons in an atom’s nucleus, then this is already the ninety-second element. Secondly, this number contains information on how many electrons this element has. In other words, ten electrons fly around the nucleus of the tenth element atom.
Each element has its own name. This name is displayed in the left corner of the cell at the bottom. Naturally, it is inconvenient to use the full names of chemical elements in scientific notes. Therefore each element has its own symbol, a short designation. The symbol for each element is indicated in the upper left corner of a cell.
Each element has its own atomic mass measured in atomic mass units and is indicated above the element name.
Here you can find more interesting facts for kids about atoms, their structure and properties.
The principle by which elements in the table are ordered
Periods
In the periodic table, chemicals are arranged in a particular order: from left to right as their atomic masses grow. All of them are combined into periods and groups. The table consists of seven periods and eighteen groups.
Periods are horizontal rows in the table.
Elements that belong to the same period show the following patterns with an increase in their serial number:
- electronegativity increases,
- metallic properties decrease, non-metallic properties increase,
- atomic radius decreases.
The periods in the table are divided into small and big ones. Small periods are periods that contain a small number of elements. These are the first, second, and third periods. The first consists of 2, the second and third of 8 elements.
All other periods are large periods — the fourth and fifth consist of 18 elements, the sixth of 32, the seventh of 24.
At the very bottom of the table, there are substances that were named lanthanides and actinides.
The periodic table contains ten rows. Small periods consist of one row. Large periods contain two rows. In the seventh period, there is one row.
Each big period consists of odd and even rows. Even rows contain metals. Odd rows contain non-metals.
The periodic table begins with hydrogen, the first chemical element, and ends today with the 118th, oganesson. Scientists argue that the table is not complete. There is an active search for the 119th element.
Groups
A group is a vertical column in the periodic table that defines the basic physical and chemical properties of elements. Substances belonging to the same group have similar chemical characteristics and demonstrate the same pattern in the change in their properties as the atomic number increases.
Elements that belong to the same group show the following patterns from top to bottom:
- the radius of the atom of elements within the same group increases,
- metallic properties are improved, and the non-metallic ones are weakened,
- electronegativity drops.
The difference between metals, metalloids, and non-metals
All chemical elements, depending on their chemical and physical properties, can be divided into three types: metals, metalloids, non-metals.
Characteristics of metals:
- good electrical and thermal conductivity,
- the ability to reflect light (bright appearance),
- high melting point (remain solid at normal ambient conditions, except for mercury),
- plasticity and pliability.
Examples of metals are copper, aluminum, gold.
Non-metals occur naturally in three states: gas (such as hydrogen), liquid (such as bromine), and solids (such as phosphorus). Their properties are:
- it is difficult for them to pass heat, and it is hard to be an electrical conductor,
- diverse appearance (elements with low density and brightness),
- significantly lower melting point compared to metals,
- brittleness and fragility.
Metalloids have mixed properties of metals and non-metals (for instance, silicon). These are their main features:
- average heat and electrical conductivity,
- the appearance can be similar to metals or non-metals,
- they differ in melting point, density, color, and shape.
Interesting facts about the Periodic table
The table of chemical elements of the famous chemist D. Mendeleev is a real breakthrough in chemistry, which the whole world could see in the spring of 1869. The Russian chemist was able to group and arrange knowledge about each chemical element in the form of a practical table, which is now familiar to every student. The periodic table became the basis for the rapid development of such a difficult and at the same time fascinating subject, while its appearance is shrouded in myths and legends.
One of the most common is the misconception that the idea of the system came to the scientist in a dream. The chemist himself refuted this legend and claimed that he had been working on its development for many years. To organize the elements, he wrote them all down on separate cards and many times tried to combine them, arranging the cards in a row based on similar properties.
The legend of a prophetic dream appeared because the scientist himself worked on the classification of all chemicals for days, occasionally taking a break for a couple of hours of sleep. But only many years of hard work and the innate talent of Mendeleev gave the result in the form of a well-known table and brought the promising scientist fame all over the world.

new engaging articles